
PLEASE READ THIS IN CONJUNCTION WITH THE ELECTROLYIC COMPATABILITY TABLE.
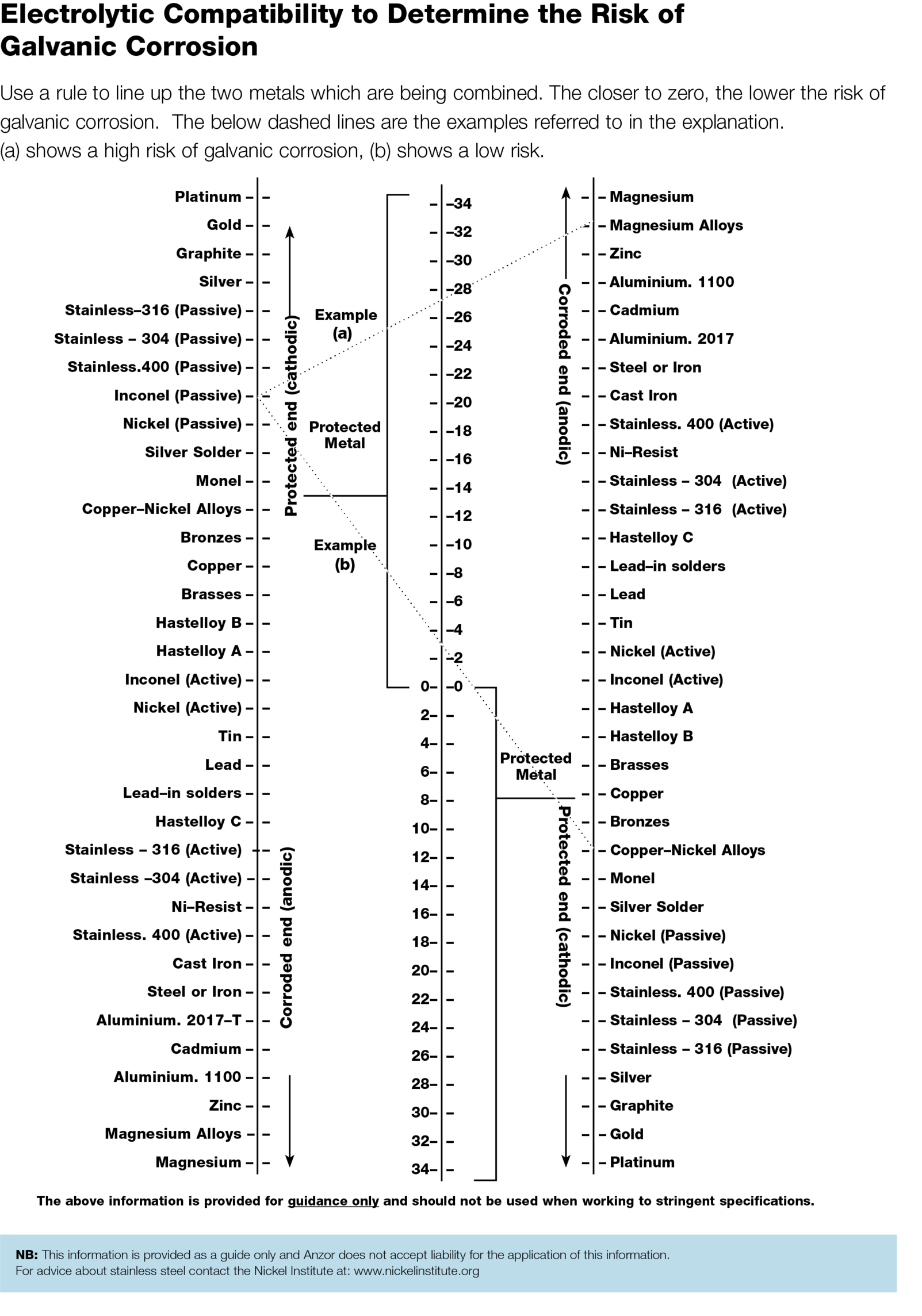
When two dissimilar metals or two similar metals which differ only slightly in chemical composition are in contact in the presence of moisture (e.g. a damp or humid atmosphere) electrolytic or galvanic corrosion can occur.
This is caused by the generation of a minute current that causes one of the metals to be eaten away.
Metals furthest apart in an electrolytic series will corrode most rapidly. The Electrolytic compatability table shows the possible extent of electrolytic corrosion for comparison purposes, as well as the differences in corrosion rates for different metal combinations.
A straight line is drawn between the two metals being considered; one metal selected from the left hand list and the other from the right hand list. The centre column number scale represents the degree of possible corrosion activity.
A large number indicated high possibility of electrolytic activity, whilst a low number indicated a better combination for resisting electrolytic corrosion. The left and right hand material columns also indicate which metal will be protected and which will be attacked by corrosion.
Example:
A greater corrosion rate than indicated can result if a small Anode is in contact with a large Cathode since the electrical current density is increased at the Anode.
Where it is not possible to use materials that will resist corrosion then use a protective agent such as Tef Gel or use rubber/nylon washers If the materials are likely to be exposed to particularly corrosive conditions.
To avoid galvanic corrosion, the dissimilar metals need to be isolated from each other and not in contact. Nylon or EPDM washers can be used as well as Tef-Gel.
The contact between dissimilar metals in the presence of an electrolyte, like salty water, can create galvanic corroson. The chart shows to what extent this can occur.
By using such products as nylon/epdm washers or tef gel this can be prevented or diminished.
NB: This information is provided as a guide only and Anzor does not accept liability for the application of this information.
For advice about stainless steel, contact the Nickel Institute
Build Your Deck Faster With Our Decking Screw Calculator
Super Duplex 2507 Stainless Steel for Engineering & Manufacturing
To receive useful info and product updates add your details below